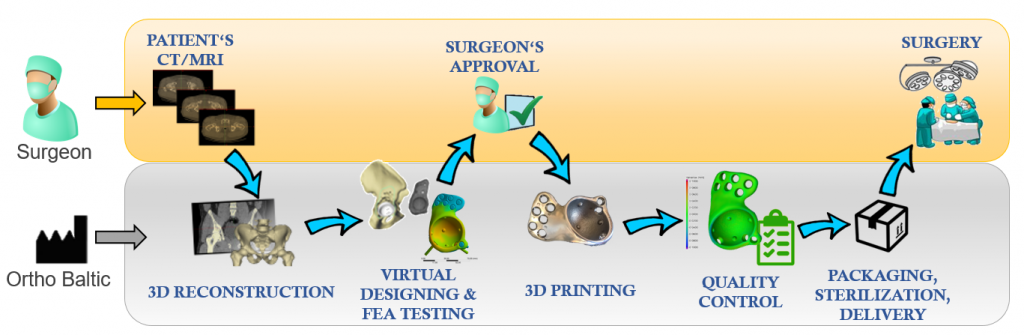
Obtaining Patient's data
Obtaining Patient's data Anatomical segmentation
Anatomical segmentation Pre-surgical Planning
Pre-surgical Planning Surgeon's Approval
Surgeon's Approval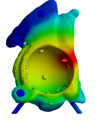 Final FEA Testing
Final FEA Testing 3D Printing / 5+1 axis milling
3D Printing / 5+1 axis milling Quality Control
Quality Control Sterilization & Packaging
Sterilization & Packaging
Obtaining data and requirements for a patient-specific implant
The process begins with the submission of the patient’s medical data and design specifications of the patient-specific implant to Ortho Baltic. The surgeon provides data from the patient’s radiological examination. A computed tomography (CT) scan is usually sufficient; however, if the surgeon requests to not be limited to the personalisation of the implant’s topology and its primary fixation solutions, and wants the endoprosthesis to be biomechanically adjusted to the adjacent joints that have been already adapted to the structural changes of the patient’s skeleton, then data from other radiological examinations are necessary (for instance, plain radiogram of a lower limb in two profiles that would show the position of all the joint axes of the lower limb with respect to one another). The data of the patient’s radiological examination(s) are accepted only in DICOM file format. The possible ways of data submission are following:- transferring using PACS servers;
- sending using other virtual file storage / transfer servers;
- data burned to a digital storage device (CD, DVD, or USB key) sent via mail.
Anatomical segmentation of the patient-specific model
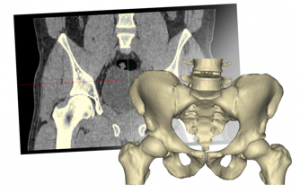
From the patient’s 2D images of the radiological examination(-s), 3D reconstruction engineers, working with a radiologist if necessary, and using certified Mimics® Medical software, perform 3D reconstruction, i.e., they reconstruct a virtual patient-specific anatomical model(-s) that is then used to design the patient-specific implant.Primary design of a patient-specific implant and surgical guides
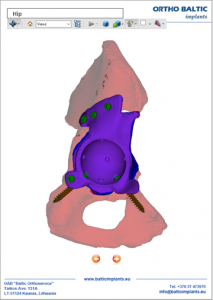
According to the patient-specific anatomical model and the requirements described in the Order specifications form, primary designs of patient-specific implant and single-use surgical guides are carried out. A non-destructive testing of the implant is also conducted via the Finite Element Analysis software ANSYS® (analysis of the implant structural characteristics). If needed, the primary design may be refined using interactive communication with the surgeon.Presurgical planning / specification of the requirements for the patient-specific implant and surgical guides
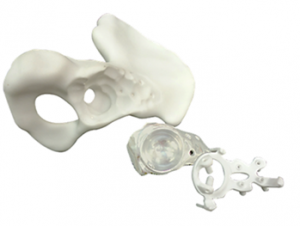
The following patient-specific models resulting from the previous steps are provided for Presurgical Planning (PP): anatomical model, models of the implant and surgical guides. PP models may be provided in two ways:- a virtual form (using a 3D pdf file format) or
- materialized from PA 2200 (Nylon-12) biocompatible material using 3D printing SLS technology
Surgeon’s confirmation of the suitability of the design and set of the patient-specific implant
If the results of the Presurgical Planning (PP) are conclusive, the surgeon fills out the Presurgical Planning Protocol (PPP) and confirms by signature that the surgical procedure plan, patient-specific implant design and set accompanying the implant are suitable for one particular patient (with his / her specific clinical condition).Final testing of the patient-specific implant design
When the surgeon signs the PPP document, the final non-destructive testing of the virtual implant design is carried out employing Finite Element Analysis software ANSYS®.Manufacturing technologies and materials used
The following materials and manufacturing technologies are used to produce patient-specific implants:- To manufacture patient-specific implants, we use the additive manufacturing technology DMLS (Direct Metal Laser Sintering) and medical Grade 5 titanium alloy Ti6Al4V powder, which is poured in 30-micron layers and sintered with an optical laser;
- Some components of patient-specific implants (for instance, the component of the fossa for the temporomandibular joint endoprosthesis) are manufactured from ultra-high molecular weight polyethylene (UHMWPE) using a 5+1 axis milling technology;
- Single-use patient-specific surgical guides are printed from biocompatible plastic (PA 2200, also known as Nylon-12) using additive manufacturing technology SLS (Selective Laser Sintering).
Medical device verification and validation (Quality control)
Patient-specific implants that come with patient-specific surgical guides are class IIb to III medical devices; therefore, their validation and verification are performed in all the product development stages in comply with the requirements of the ISO 13485 standard. During the final product quality control, the implant undergoes analysis of the geometry, surface roughness, internal defects and chemical composition.Medical device sterilisation and packaging
The cleaning, washing, disinfection and packaging of patient-specific implants and surgical instruments are carried out in the company’s certified ISO Class 7 cleanroom.
All the implants containing lattice structure in their construction are packaged and sent to a certified institution in Germany for gamma sterilisation. All the other medical devices without lattice structure are sterilized by gamma rays only by request. Otherwise, medical devices are delivered to the healthcare institution prepared for sterilisation in the autoclave: cleaned, disinfected and packaged.